Each year in the United States there are more than 1M patients that try steroid injections to relieve the pain associated with a disc herniation in their lower back. Data released by the U.S. Centers for Medicare and Medicaid in 2014, suggested this number could be as high as 5M patients per year. Another 250,000 patients have exhausted non-surgical treatment and opt for surgery. Minimus Spine believes that it can benefit these patients with a single injection and reduce the number of patients that ultimately need surgery.
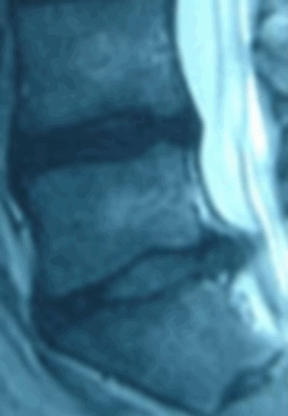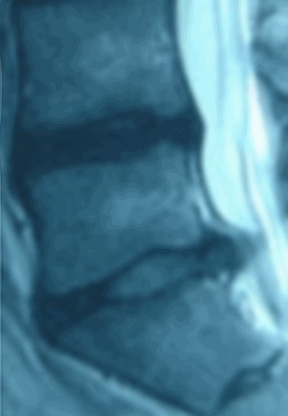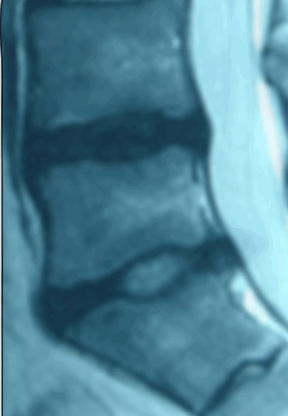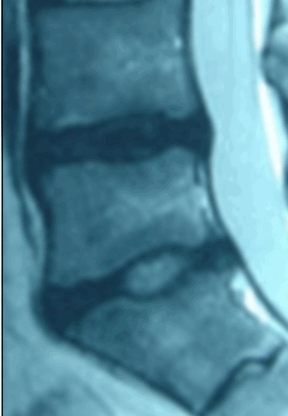 Ozone has been used outside the United States to treat disc herniations for over 15 years and there are over 40 peer-reviewed papers documenting over 8,000 patients. Treatment with ozone is not FDA approved and the Triojection® System is not available in the United States.
Ozone has been used outside the United States to treat disc herniations for over 15 years and there are over 40 peer-reviewed papers documenting over 8,000 patients. Treatment with ozone is not FDA approved and the Triojection® System is not available in the United States.
Minimus Spine developed the Triojection System to elevate the standard for performing an ozone injection. Triojection is a patented system that creates ozone and measures its concentration, within a sterile, single-use syringe cartridge. Triojection has advantages in terms of sterility and confidence in the concentration of gas delivered to the patient, as well as material compatibility. All of these improvements are vital to the advancement of this technique.
Minimus Spine and the Triojection system have received the necessary certifications to commercialize Triojection in Europe (ISO 13485 Certification and EC Certificate). British Standards Institute is Minimus Spine’s Notified Body.
Minimus Spine seeks to improve patient care and cut health care costs by reducing the number of steroid injections and surgeries.